Today is all about everyone's theoretical relative, the atom.
Now, you may be thinking to yourself, what exactly is an atom?
An atom is the tiniest little unit of matter that makes up an element. They are called "The building blocks of the universe.", since all matter contains atoms, meaning that everything has atoms, including humans, animals, the soil of which makes up the earth and the air we breathe. You literally cannot avoid atoms at all, they are there interacting with you without your knowledge (which sounds a bit strange, but you get the point).
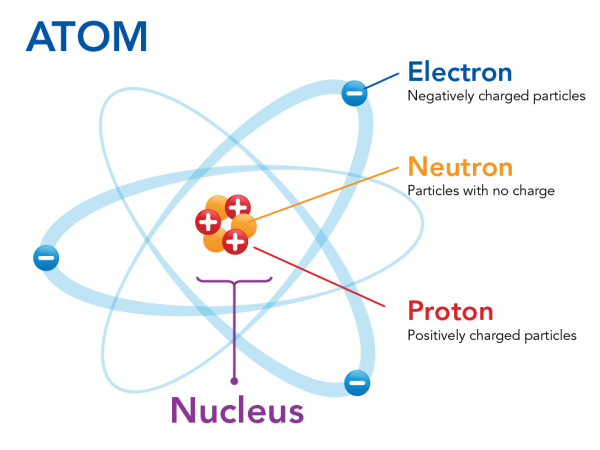
In the image above, it shows the buildings blocks which make up an atom. Now, don't get confused. Atoms might be the smallest unit that make up matter, but they're not the tiniest things in science. That title goes to the particles that make up an atom.
In an atom, there are three main particles that make an atom:
- Protons
- Neutrons
- Electrons
Protons contain a positive electrical charge, Neutrons contain none and Electrons contain a negative electrical charge. Both the protons and neutrons stay in the center of the atom, sitting comfortably and becoming the nucleus, while the electrons move around freely. Now, in a normal diagram of an atom, they are shown to revolve around the nucleus. However, this is just a way to easily represent the electrons and how they work, while in truth, the electrons shoot around freely and move in an uncontrolled fashion.
Back to charges. An atom usually has no electrical charge since the positive charge of a proton is cancelled out by the negative charge of the electron, and since the neutron has no charge at all, it remains neutral.
Back to electrons. You may not realize how fast electrons travel, but they travel stupid fast, approximately 2,200km/s.
In regards to how much of an atom is empty space, technically speaking, is none. Theoretically speaking, there is no such thing as pure empty space, since electrons fill in that role of filling in that space. But if we're going off of approximate theories, then an atom is filled with about 99.996% empty space (rounding off to three decimal places).
Anyway, that's me.
Stay safe boys.
-M.V
Good job Mase! Its the weirdest thing, the Atom. It exists, but is mostly empty quantum space.
ReplyDelete